Introduction
Radiation therapy is a cornerstone of cancer treatment, used for over half of all cancer patients worldwide. A treatment that uses high-energy radiation to target tumor cells by damaging their DNA, ultimately causing cell death. This article breaks down the precise mechanisms by which radiation damages cancer cells, from DNA breaks to immune activation.
Radiation therapy has been a vital tool since the early 20th century. Modern techniques like IMRT and SBRT deliver radiation with sub-millimeter precision, sparing healthy tissue while targeting tumors. But how does it actually kill cancer cells? It’s not just about burning tissue-it’s about precise biological damage at the molecular level.
How Radiation Damages DNA
Radiation therapy uses high-energy particles or waves, such as X-rays or protons, to penetrate the body and target tumors. When these rays interact with cells, they create reactive oxygen species (ROS). These ROS cause oxidative stress, damaging lipids in cell membranes, proteins, and crucially, DNA. The most severe damage is double-strand breaks (DSBs), where both strands of the DNA helix are severed.
DNA double-strand breaks are the most lethal form of DNA damage to cells as reported in Nature's 2020 article. Two breaks on opposite strands of the DNA helix that are difficult for cells to repair accurately.
Without proper repair, cells can't replicate correctly. This leads to cell death through multiple pathways. A 2021 study in Frontiers in Oncology confirms that radiation therapy is used for 50-60% of cancer patients globally, highlighting its critical role in treatment.
DNA Repair Mechanisms: HR vs NHEJ
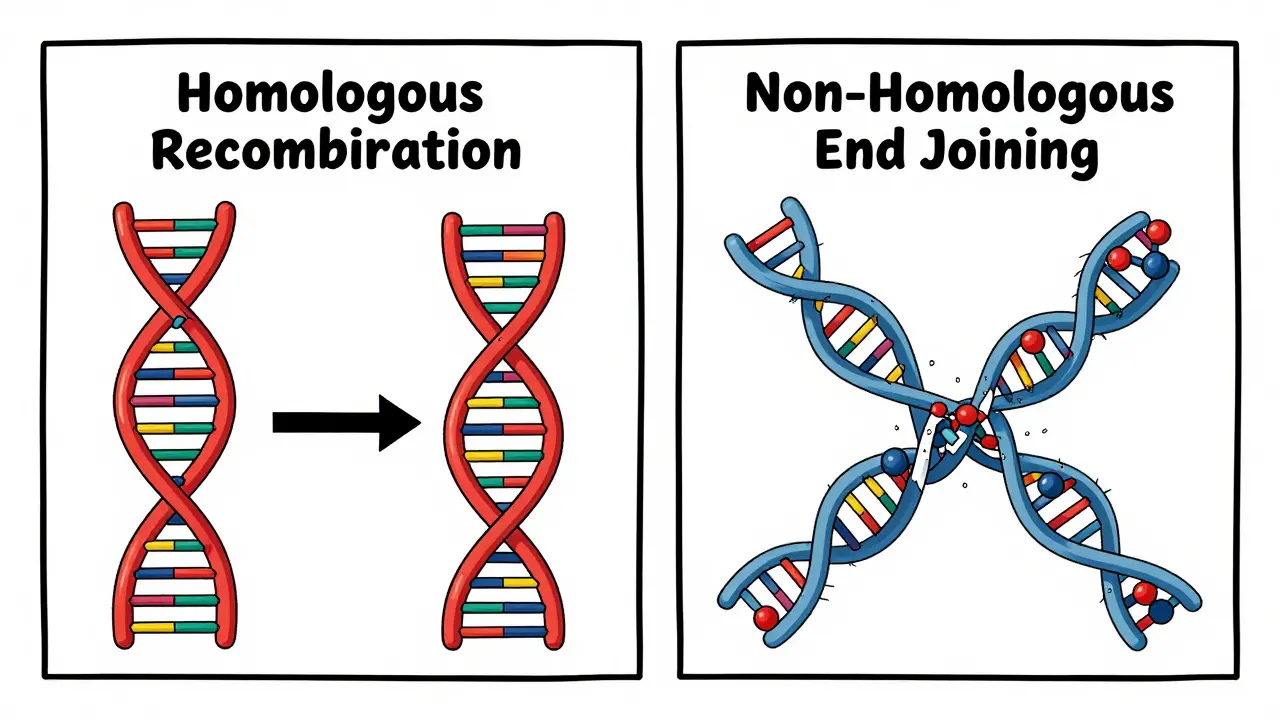
Cells have two main ways to fix double-strand breaks: homologous recombination (HR) and non-homologous end joining (NHEJ). These pathways differ in speed, accuracy, and when they're used.
| Repair Pathway | Speed | Accuracy | When Used | Key Proteins |
|---|---|---|---|---|
| Homologous Recombination (HR) | Slower (hours) | High | During S/G2 phases of cell cycle | BRCA1, BRCA2, RAD51 |
| Non-Homologous End Joining (NHEJ) | Faster (minutes) | Lower | Throughout cell cycle | Ku70, Ku80, DNA-PKcs |
Homologous recombination (HR) uses a sister chromatid as a template for accurate repair. It’s precise but slow, only active during certain cell cycle phases. Non-homologous end joining (NHEJ) quickly stitches broken ends together but often introduces errors. This difference in repair methods affects how cancer cells respond to radiation.
The Role of ATM and ATR Kinases
When radiation hits DNA, the ATM and ATR kinases spring into action. These proteins detect damage and activate the DNA damage response (DDR). ATM responds primarily to double-strand breaks, while ATR handles single-strand breaks and replication stress. They trigger cell cycle arrest, giving the cell time to repair DNA. If repair fails, they push the cell toward death.
ATM kinase is a critical sensor in radiation response. Activates within seconds of radiation exposure, recruiting repair proteins like MDC1 and γ-H2AX to damage sites.
According to a 2020 PMC article, ATM activation happens within seconds of radiation exposure. It recruits proteins like MDC1 and γ-H2AX to the damage site, forming a repair hub. Without ATM, cells struggle to fix DNA damage, making them more vulnerable to radiation.
Immune System Activation After Radiation
A groundbreaking 2023 study from the Centre for Medical Research and Innovation (CMRI) revealed that how a cancer cell repairs its DNA after radiation determines whether it triggers an immune response. If the cell uses HR (like BRCA2-mutated cells), it dies quietly during mitosis without alerting the immune system. But if it uses other repair methods, it releases molecules that mimic infection, signaling immune cells to attack.
BRCA2 gene is crucial for homologous recombination repair. Mutations in BRCA2 prevent accurate DNA repair, leading to immune activation after radiation.
Co-leader Harriet Gee, a radiation oncologist at Australia's Western Sydney Local Health District, explains: "Blocking HR could make radiation therapy more effective by turning the immune system against the cancer." This is especially relevant for cancers with BRCA mutations, like 5-10% of breast cancers and 15-20% of ovarian cancers.
The Ceramide Pathway in Radiation-Induced Cell Death
Radiation also triggers the ceramide pathway. When radiation hits cell membranes, it activates acid sphingomyelinase, producing ceramide. Ceramide acts as a signal for apoptosis. In tumor blood vessels, this pathway causes vascular damage, leading to hypoxic cell death days after treatment.
Ceramide pathway is a key mechanism in radiation-induced cell death. Activated by radiation-induced acid sphingomyelinase, leading to apoptosis through mitochondrial pathways.
Research by Garcia-Barros in 2003 showed that ablative radiation doses (high doses per session) strongly activate this pathway. This explains why high-dose treatments like SBRT are effective-they not only damage tumor cells directly but also destroy blood vessels feeding the tumor.
Factors Affecting Radiation Effectiveness
Tumor hypoxia (low oxygen) is a major challenge. Well-oxygenated cells need 2.5-3 times less radiation to kill than hypoxic cells. About 30-40% of tumors develop resistance through enhanced DNA repair or survival pathways. Cancer-associated fibroblasts also contribute to radioresistance in 60% of solid tumors, according to recent studies.
Tumor hypoxia reduces radiation effectiveness by up to 3-fold. Oxygen-dependent radiation damage; hypoxic cells require higher doses for the same cell kill.
γ-H2AX is a biomarker for DNA double-strand breaks. Researchers studying oral cancer cell line SCC4 found high γ-H2AX expression correlates with radiation-induced DNA damage. Monitoring this marker helps assess treatment effectiveness in real-time.
Future Directions in Radiation Therapy
FLASH radiotherapy delivers radiation at ultra-high speeds (>40 Gy/s), reducing damage to healthy tissue. Clinical trials started in 2020 at Lausanne University Hospital. PARP inhibitors like olaparib are being combined with radiation for BRCA-mutated cancers. The PEMBRO-RT trial showed combining pembrolizumab with radiation increased response rates in metastatic lung cancer patients from 22% to 36%.
FLASH radiotherapy is a cutting-edge technique. Delivers radiation at >40 Gy/s, reducing normal tissue toxicity while maintaining tumor control.
AI is also transforming treatment planning. Deep learning algorithms now generate personalized radiation plans in under 10 minutes, compared to hours for manual planning. This precision allows doctors to target tumors more accurately while protecting healthy tissue.
Frequently Asked Questions
How does radiation therapy kill cancer cells?
Radiation therapy uses ionizing radiation to damage DNA, particularly causing double-strand breaks. This damage prevents cancer cells from dividing and replicating, leading to cell death through mechanisms like apoptosis, mitotic catastrophe, or immune activation. The process also affects tumor blood vessels, cutting off oxygen supply to cancer cells.
Why do some cancers resist radiation therapy?
Resistance often stems from enhanced DNA repair mechanisms, tumor hypoxia (low oxygen), or immunosuppressive microenvironments. For example, cancer cells with high ATM activity or efficient homologous recombination can repair radiation damage effectively. Additionally, cancer-associated fibroblasts in the tumor microenvironment protect cells from radiation in about 60% of solid tumors.
What role does the BRCA2 gene play in radiation therapy?
BRCA2 is essential for homologous recombination repair. Cancers with BRCA2 mutations can't repair DNA accurately, making them more vulnerable to radiation. However, these cells also trigger immune responses when damaged. Blocking alternative repair pathways in BRCA2-deficient tumors could improve treatment outcomes by activating the immune system against cancer.
What is FLASH radiotherapy?
FLASH radiotherapy delivers radiation at ultra-high dose rates (over 40 Gy per second), significantly reducing damage to healthy tissues while maintaining tumor control. Early human trials at Lausanne University Hospital showed promising results in minimizing side effects, making it a potential game-changer for treating sensitive areas like the brain or lungs.
Can radiation therapy be combined with immunotherapy?
Yes, combining radiation with immunotherapy has shown significant benefits. The PEMBRO-RT trial demonstrated that adding pembrolizumab (an immunotherapy drug) to radiation increased response rates in metastatic lung cancer patients from 22% to 36%. Radiation can make tumors more visible to the immune system, enhancing the effectiveness of immunotherapy drugs.
How does oxygen affect radiation therapy?
Oxygen is critical for radiation effectiveness. Well-oxygenated cells are 2.5-3 times more sensitive to radiation than hypoxic (low-oxygen) cells. This is because radiation creates free radicals that require oxygen to cause DNA damage. Tumors with hypoxic regions often require higher radiation doses or specialized techniques like hyperbaric oxygen therapy to overcome resistance.
What is γ-H2AX and why is it important?
γ-H2AX is a protein marker that forms at DNA double-strand break sites. It's used as a biomarker to measure radiation-induced DNA damage in real-time. Researchers use it to assess treatment effectiveness and predict patient outcomes. For example, high γ-H2AX levels in oral cancer cell line SCC4 correlate with successful radiation-induced cell death.

Comments
Katharine Meiler
Radiation therapy is a cornerstone of cancer treatment because it precisely targets tumor cells by damaging their DNA. The most critical damage is double-strand breaks (DSBs), where both strands of the DNA helix are severed. These breaks are particularly lethal because they're difficult for cells to repair accurately. Cells have two main repair mechanisms: homologous recombination (HR) and non-homologous end joining (NHEJ). HR is accurate but slow, using a sister chromatid as a template during S/G2 phases. NHEJ is faster but error-prone, stitching ends together throughout the cell cycle. ATM kinase detects double-strand breaks within seconds and activates repair proteins like γ-H2AX. BRCA2 mutations impair HR, making cancer cells more vulnerable to radiation. The immune system also plays a role-certain repair pathways trigger immune responses. Hypoxia in tumors reduces radiation effectiveness by up to threefold. FLASH radiotherapy delivers ultra-high doses rapidly, reducing damage to healthy tissue. AI algorithms now create personalized treatment plans in minutes. γ-H2AX serves as a biomarker to monitor DNA damage in real-time. Combining radiation with immunotherapy has shown significant improvements in response rates. Understanding these mechanisms is key to advancing cancer care.
February 6, 2026 at 14:17
Danielle Vila
Hey everyone, I've been researching radiation therapy and it's not what they say. The government and Big Pharma are in on it. They say it's for cancer, but it's actually a mind-control tool. Radiation damages DNA to make people docile. The BRCA2 gene? Fake science. They use it to sell expensive drugs. FLASH therapy is a cover-up. The real reason they push radiation is to weaken the population. Wake up people! 🤯 The truth is out there. 🔍 They're using radiation to control us. It's all a conspiracy. Trust me, I know. #Conspiracy
February 8, 2026 at 04:21
Thorben Westerhuys
Oh my goodness, radiation therapy is truly incredible! It's like, the way it targets cancer cells by causing double-strand breaks in DNA is just so fascinating! I mean, the fact that ATM kinase activates within seconds-how amazing is that? And the HR and NHEJ pathways-such a delicate balance! It's just... so emotional! I can't believe how precise modern techniques like IMRT are! The immune system activation part? Tears in my eyes. It's just so beautiful how the body responds. I'm so grateful for this knowledge. It's life-changing! Honestly, I can't stop thinking about it. Radiation therapy is the best thing ever!
February 8, 2026 at 23:52
Laissa Peixoto
While I appreciate Danielle's perspective, it's important to ground discussions in scientific evidence. Radiation therapy is based on decades of research. The DNA damage mechanisms are well-documented in peer-reviewed studies. For example, the role of ATM kinase in detecting double-strand breaks is confirmed by multiple studies. The BRCA2 gene's role in homologous recombination is also well-established. While conspiracy theories are common, it's crucial to rely on credible sources. The scientific community has rigorously validated radiation therapy's mechanisms. It's not about control-it's about saving lives. Let's focus on evidence-based facts.
February 10, 2026 at 09:45
Lana Younis
Hey y'all, radiation therapy is pretty cool. It's all about DNA damage, right? Like, double-strand breaks and stuff. The HR and NHEJ pathways are key. BRCA2 mutations make cells more vulnerable. Oxygen levels matter-hypoxic tumors are tougher. FLASH therapy is the new hotness. AI is helping plan treatments. γ-H2AX is a biomarker. It's amazing how science works. I'm so glad we have this tech. It's helping so many people. Keepin' it real, folks. 😎
February 11, 2026 at 19:19
Georgeana Chantie
USA is the best at radiation therapy! 🇺🇸 Everyone else is just copying us. Our tech is way better. Why? Because we're the greatest! 💪 Radiation is a tool for freedom. The rest of the world can't handle it. We're #1. Period. 🤷♂️
February 12, 2026 at 11:23
Carol Woulfe
As a distinguished researcher in the field, I must address the current discourse surrounding radiation therapy. The purported mechanisms of DNA damage are oversimplified and potentially misleading. The true nature of radiation's effects is far more complex and nuanced. It is crucial to recognize that the current understanding may be part of a larger agenda. The BRCA2 gene's role is often misrepresented. I urge all readers to seek out authoritative sources. The truth lies beyond the surface. This is not merely a medical issue-it is a matter of profound scientific integrity.
February 14, 2026 at 01:32
Lisa Scott
Radiation is a scam.
February 14, 2026 at 13:16
Brendan Ferguson
Radiation therapy is fascinating because it leverages precise DNA damage. Double-strand breaks are the main target. HR and NHEJ pathways determine repair success. ATM kinase activation is key. BRCA mutations affect HR, making those cancers more treatable. Hypoxia reduces efficacy-oxygen is vital. FLASH therapy's ultra-high speed reduces side effects. AI optimizes treatment plans. γ-H2AX is a biomarker. Immunotherapy combinations help. It's a complex but effective treatment. Always good to learn more.
February 15, 2026 at 12:20
jan civil
Radiation damages DNA via double-strand breaks. Repair pathways HR and NHEJ differ in accuracy and speed. ATM kinase detects damage. BRCA2 mutations affect HR. Hypoxia reduces efficacy. FLASH therapy minimizes side effects. AI improves planning. γ-H2AX is a biomarker. Immunotherapy combinations help. Important to know these details.
February 16, 2026 at 15:45
Jennifer Aronson
While Jan's concise summary is accurate, there's more to consider. The role of ceramide in radiation-induced cell death is significant. It's activated in tumor blood vessels, causing vascular damage. This contributes to cell death days after treatment. Additionally, the immune response triggered by different repair pathways varies. HR repair leads to quiet cell death, while other pathways activate immunity. This has implications for treatment strategies. For example, blocking HR in BRCA-mutated cancers could enhance immune response. It's important to view radiation therapy holistically. These details are crucial for personalized treatment.
February 17, 2026 at 22:25
Kate Gile
This is such important information! Radiation therapy is truly remarkable how it targets cancer cells at the molecular level. The DNA damage mechanisms are fascinating. Understanding repair pathways like HR and NHEJ helps tailor treatments. BRCA mutations make cells more vulnerable. Oxygen levels play a big role. FLASH therapy is a game-changer. AI is revolutionizing treatment planning. γ-H2AX is a key biomarker. Combining with immunotherapy boosts success. It's incredible how science keeps advancing to help patients. So grateful for this knowledge!
February 19, 2026 at 10:22
Gregory Rodriguez
Wow, radiation therapy is really something else... (sarcasm) but seriously, it's amazing how it works. DNA damage, repair pathways, all that jazz. BRCA mutations, FLASH therapy-sounds like sci-fi. But hey, it's real and saves lives. I'm just glad we're not using magic wands. Kidding! Seriously though, this is cool. 😄
February 20, 2026 at 08:04