Topical Treatment Decision Guide
Personalized Treatment Recommendation
Answer a few questions about your condition and preferences to get a tailored recommendation for your skin treatment.
Recommendations
Recommended Treatment
Strengths
Potential Side Effects
Quick Takeaways
- Imiquad contains 5% imiquimod, ideal for actinic keratosis and superficial skin cancers.
- Major alternatives include 5‑fluorouracil, diclofenac gel, ingenol mebutate, cryotherapy and photodynamic therapy.
- Imiquad’s side‑effects are mostly local skin reactions; systemic issues are rare.
- Cost varies widely - Imiquad is mid‑range, while generic 5‑fluorouracil is usually cheaper.
- Choosing the right option depends on lesion type, patient tolerance, and convenience.
When it comes to treating actinic keratosis, superficial basal cell carcinoma, or genital warts, Imiquad Cream is often mentioned alongside a handful of other topical options. This Imiquad cream comparison breaks down how it stacks up against the most common alternatives, covering everything from how the drugs work to side‑effects, treatment schedules, and price. By the end you’ll know which cream or procedure fits your skin condition, lifestyle, and budget best.
What Is Imiquad Cream?
Imiquad Cream is a prescription‑only topical medication that contains 5% imiquimod, an immune‑modulating agent. First approved in the early 2000s for genital warts, it later earned labels for actinic keratosis (AK) and superficial basal cell carcinoma (sBCC). The cream is applied once daily, five nights a week, for a typical course of four weeks for AK and six weeks for sBCC.
How Imiquad Works
Imiquimod is not a classic cytotoxic drug. Instead, it triggers the body’s innate immune system by binding to Toll‑like receptor 7 (TLR‑7) on dendritic cells and macrophages. This activation ramps up the production of interferon‑α, tumor necrosis factor‑α, and interleukins, which together recruit killer T‑cells to the treated skin. The result is a localized immune attack that destroys abnormal keratinocytes while sparing healthy tissue.
Top Alternatives to Imiquad
Several other topical agents and procedures are used for the same indications. Below is a quick snapshot of each.
- 5‑Fluorouracil (5‑FU) - a pyrimidine analog that interferes with DNA synthesis, usually prescribed as a 5% or 0.5% cream.
- Diclofenac Gel (Solaraze) - a non‑steroidal anti‑inflammatory gel (3%) that reduces prostaglandin‑mediated proliferation.
- Ingenol Mebutate (Picato) - a diterpene ester that induces rapid cell death and a secondary immune response.
- Cryotherapy - liquid nitrogen applied to the lesion for 10‑15 seconds, causing freeze‑thaw cell destruction.
- Photodynamic Therapy (PDT) - a two‑step process using a photosensitizer (e.g., aminolevulinic acid) and blue/red light to generate reactive oxygen species.
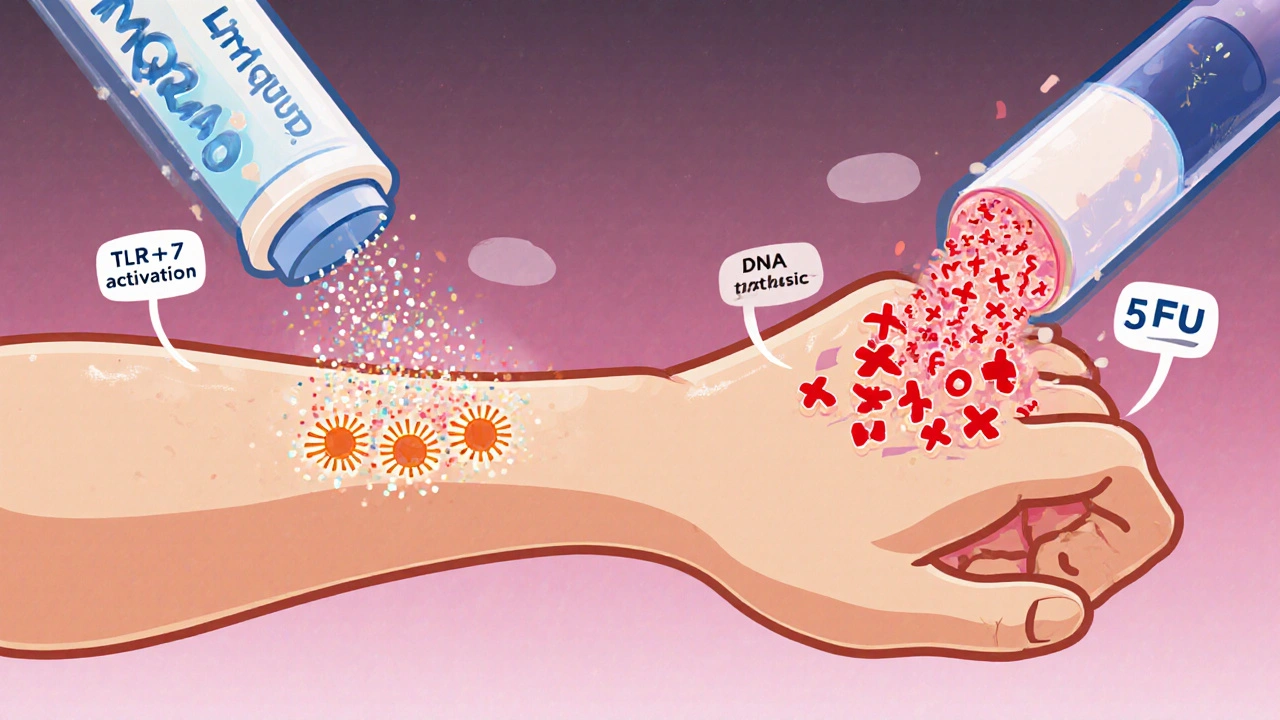
Comparing Mechanisms of Action
Understanding how each treatment attacks abnormal skin cells helps predict efficacy and side‑effects.
- Imiquad (Imiquimod) - immune activation via TLR‑7.
- 5‑FU - direct DNA synthesis inhibition; leads to apoptosis of rapidly dividing cells.
- Diclofenac - blocks COX‑2, reducing inflammatory mediators that drive keratinocyte growth.
- Ingenol Mebutate - causes rapid tumor necrosis plus a secondary immune response.
- Cryotherapy - physical destruction through extreme cold.
- PDT - photo‑activated oxidative damage within treated cells.
Application Schedules & Treatment Duration
Each option comes with a different regimen, which can affect patient compliance.
| Product / Procedure | Typical Duration | Frequency | Typical Course Length |
|---|---|---|---|
| Imiquad (Imiquimod 5%) | Apply nightly | 5 nights/week | 4‑6 weeks |
| 5‑Fluorouracil 5% | Apply twice daily | Every day | 2‑4 weeks |
| Diclofenac Gel 3% | Apply twice daily | Every day | 6‑12 weeks |
| Ingenol Mebutate | Single‑day application | One‑time | 1‑2 days (post‑treatment inflammation lasts ~1 week) |
| Cryotherapy | Single session per lesion | As needed | Usually 1‑2 visits |
| Photodynamic Therapy | Two‑day protocol | Two sessions 1‑2 weeks apart | ~2‑3 weeks total |
Side‑Effect Profiles
Local skin irritation is the most common complaint for all topical agents, but the intensity and type differ.
- Imiquad: erythema, crusting, itching, and occasional ulceration. Systemic flu‑like symptoms are rare.
- 5‑FU: intense redness, pain, crusting, and possible ulcer formation; discomfort often peaks at week 2.
- Diclofenac: mild to moderate erythema and scaling; generally better tolerated than 5‑FU.
- Ingenol Mebutate: brief burning sensation followed by localized inflammation that can look dramatic but usually resolves in a week.
- Cryotherapy: transient pain during freezing, blistering, and occasional hypopigmentation.
- PDT: photosensitivity, acute pain during illumination, and post‑treatment erythema that may last several days.
Cost Considerations (2025 UK Prices)
Price can be a deciding factor, especially for long‑term courses.
| Product / Procedure | Approx. Cost | Insurance Reimbursement |
|---|---|---|
| Imiquad (prescription) | £85‑£110 per 5‑g tube | Partial NHS coverage for high‑risk lesions |
| 5‑Fluorouracil (generic) | £30‑£45 per 5‑g tube | Typically covered |
| Diclofenac Gel (Solaraze) | £70‑£90 for 30‑g tube | Limited NHS reimbursement |
| Ingenol Mebutate (Picato) | ≈£150 per 0.015‑g sachet | Rarely covered |
| Cryotherapy (clinic visit) | £70‑£120 per session | Usually covered for cancer‑related lesions |
| Photodynamic Therapy | £200‑£350 per treatment course | Variable, often specialist‑dependent |

Choosing the Right Option: Decision Guide
Below is a quick decision flow you can use while discussing with your dermatologist.
- Identify the lesion type. AK, sBCC, or genital warts each have preferred first‑line agents.
- Assess tolerance for skin irritation. If you have low pain tolerance, diclofenac or a short‑duration option like ingenol may suit you better.
- Consider treatment length. For busy schedules, a single‑day application (ingenol) or a procedural approach (cryotherapy) saves time.
- Check cost & insurance. Generic 5‑FU often wins on price, while Imiquad may be subsidized for high‑risk skin cancer.
- Review follow‑up needs. Some therapies (cryotherapy, PDT) require multiple clinic visits; topical creams are home‑based.
In many cases, dermatologists start with Imiquad or 5‑FU because they treat a broad range of lesions and have robust evidence. If the patient experiences severe local reactions, they may switch to diclofenac or opt for a procedural method.
Real‑World Patient Stories
Sarah, 62, Bristol: “I tried Imiquad for a patch of AK on my forearm. The skin reddened a lot, but after six weeks the lesion vanished. The cost was covered by my NHS plan, so I stuck with it.”
James, 48, London: “5‑FU gave me a burning feeling that made me miss work. My GP switched me to diclofenac gel, which was milder, though it took three months to see results.”
Aisha, 35, Manchester: “I needed a quick fix for a small wart. Ingenol Mebutate’s one‑time dose worked faster than the cream I’d used before.”
Key Takeaways for Clinicians
- Imiquad remains a first‑line topical for AK and sBCC because of its proven efficacy and moderate cost.
- When patients cannot tolerate the inflammation that Imiquad triggers, diclofenac or ingenol provide gentler alternatives.
- Procedural options (cryotherapy, PDT) are valuable for isolated lesions or when rapid clearance is essential.
- Always factor in NHS reimbursement rules; generic 5‑FU often minimizes out‑of‑pocket expense.
Frequently Asked Questions
Is Imiquad safe for long‑term use?
Imiquad is intended for short courses (4‑6 weeks). Long‑term, repeated cycles are possible under specialist supervision, but cumulative skin irritation can increase over time.
Can I use Imiquad on my face?
Yes, facial AK can be treated with Imiquad, but clinicians often start with a lower‑strength regimen or switch to 5‑FU to reduce the risk of severe erythema.
How does the efficacy of Imiquad compare to 5‑Fluorouracil?
Clinical trials show similar clearance rates for AK (≈80‑90%). Imiquad often produces a more visible immune response, which patients report as a sign that the treatment is working.
Are there any drug interactions with Imiquad?
Because Imiquad works locally, systemic drug interactions are rare. However, concurrent use of other topical irritants (e.g., retinoids) may amplify skin redness.
What should I do if I develop severe ulceration?
Stop the cream immediately, keep the area clean, and contact your dermatologist. They may prescribe a short course of topical steroids to reduce inflammation.
Every skin condition is unique, so the best approach blends medical evidence with personal comfort. Use this comparison as a roadmap, discuss options with your healthcare provider, and pick the treatment that aligns with both clinical need and everyday life.

Comments
sravya rudraraju
When you examine the comparative data presented for Imiquad Cream and its alternatives, several nuanced considerations emerge that merit a methodical discussion. Firstly, the immunomodulatory mechanism of imiquimod distinguishes it from cytotoxic agents such as 5‑fluorouracil, which directly impede DNA synthesis; this fundamental difference underlies divergent side‑effect profiles and therapeutic timelines. Secondly, the regimen of applying Imiquad five nights per week for a four‑to‑six‑week course aligns well with patient adherence patterns observed in clinical practice, especially when contrasted with the twice‑daily demands of 5‑FU or the protracted twelve‑week schedule of diclofenac gel. Thirdly, cost analysis reveals that while Imiquad occupies a mid‑range price tier in the United Kingdom, its partial NHS reimbursement for high‑risk lesions can offset out‑of‑pocket expenses relative to the fully covered generic 5‑FU. Fourthly, the local inflammatory response characteristic of Imiquad-manifesting as erythema, crusting, and occasional ulceration-should not be misconstrued as a treatment failure, but rather interpreted as an indicator of the intended immune activation. Fifthly, patient selection criteria must incorporate tolerance thresholds; individuals with low pain tolerance might preferentially benefit from the more temperate irritation associated with diclofenac or the single‑application convenience of ingenol mebutate. Sixthly, procedural options such as cryotherapy and photodynamic therapy offer rapid lesion clearance but necessitate clinic visits, which may be undesirable for patients seeking home‑based management. Seventhly, the evidence base supporting Imiquad’s clearance rates for actinic keratosis approximates 80‑90%, comparable to that of 5‑FU, thereby positioning it as a viable first‑line therapy. Eighthly, the potential for systemic flu‑like symptoms remains low, reinforcing the safety profile for short‑term courses under specialist supervision. Ninthly, clinicians should remain vigilant for rare but severe ulcerations, at which point therapy discontinuation and topical steroids may be warranted. Tenthly, the decision matrix must also reflect lesion morphology; facial AK may necessitate a modified dosing strategy to mitigate pronounced erythema. Eleventhly, long‑term management plans might incorporate intermittent repeat cycles of Imiquad, contingent upon patient response and dermatologic assessment. Twelfthly, integration of patient education regarding expected skin reactions can improve adherence and satisfaction. Thirteenthly, real‑world anecdotes, such as the Bristol patient who achieved lesion resolution with NHS‑covered Imiquad, underscore the practical viability of this regimen. Fourteenthly, the comparative side‑effect burden of 5‑FU, often described as intensely painful, further validates Imiquad’s balanced efficacy‑tolerability profile. Fifteenthly, the inclusion of Imiquad within multidisciplinary treatment pathways facilitates a flexible therapeutic armamentarium for dermatologists.
In summary, a comprehensive appraisal of pharmacodynamics, treatment schedules, cost considerations, and patient‑centred factors substantiates Imiquad Cream as a robust option within the contemporary dermatologic toolkit.
October 19, 2025 at 19:16
Ben Bathgate
Honestly, the whole Imiquad hype feels like another marketing gimmick when you compare it side‑by‑side with good old 5‑FU. The skin irritation is basically a badge of honor that doesn’t actually prove anything, and you’re still stuck with a pricey prescription. If you’re okay with a little burn, just grab the generic version of 5‑FU, it does the job cheaper and you won’t need a fancy cream schedule.
October 31, 2025 at 21:56
Ankitpgujjar Poswal
Listen up, if you’re battling stubborn AK and can’t afford endless doctor visits, Imiquad is the aggressive play you need. Knock that lesion out with the immune boost, don’t waste time on slow‑acting diclofenac. You’ll see redness, sure, but that’s the battle flag showing the drug is doing its job-no more excuses, just results.
November 13, 2025 at 01:36
Rakhi Kasana
While the data paints Imiquad as a balanced contender, the drama of its side‑effects can’t be ignored. Patients often describe the post‑treatment redness as a theatrical flare, turning a simple skin patch into a spotlight of inflammation. Yet, for those who cherish a narrative of triumph over lesions, this dramatic response may just be the climax they crave.
November 25, 2025 at 05:16
Catherine Viola
One must consider the hidden machinations behind pharmaceutical pricing structures, particularly the subtle subsidies that funnel Imiquimod into the NHS’s partial coverage programs. While the public narrative celebrates its efficacy, a closer inspection reveals a concerted effort by vested interests to steer clinicians toward proprietary formulations, thereby reinforcing a cycle of dependency on brand‑name therapies at the expense of more transparent, generically available alternatives.
December 7, 2025 at 08:56
Nicole Boyle
From a pharmacokinetic standpoint, Imiquimod’s TLR‑7 agonism introduces a cascade of cytokine amplification, which, when juxtaposed with the enzymatic inhibition pathways of 5‑FU, underscores a divergent therapeutic architecture. Moreover, the formulation’s vehicle-propylene glycol‑based emulsion-optimizes dermal penetration, thereby enhancing bioavailability relative to the aqueous matrix of diclofenac gel. In practice, this translates to a more robust immunologic engagement, albeit with a heightened propensity for localized erythema.
December 19, 2025 at 12:36
Caroline Keller
Imiquad? It’s just another cream that makes your skin look like it’s on fire but somehow people call it a miracle. I get it, you want quick results, but the drama is real and it’s exhausting.
December 31, 2025 at 16:16
dennis turcios
The comparison does a decent job outlining the basics, but it glosses over the patient‑specific nuances that often dictate therapy choice. For example, the tolerability of 5‑FU’s intense inflammation versus Imiquad’s moderate reaction can swing decisions based on individual pain thresholds, which isn’t fully captured here.
January 12, 2026 at 19:56
Felix Chan
Great overview, keep spreading the knowledge!
January 24, 2026 at 23:36