When you pick up a prescription, you might see two bottles on the counter: one with a well-known brand name, another with plain white labeling and no logo. Both cost different prices. You might wonder - is the cheaper one just as good? Especially when it’s labeled an authorized generic.
Here’s the truth: if you’re switching from a brand-name drug to its authorized generic, you’re getting the exact same medicine. Not close. Not almost. The same. Same pills. Same ingredients. Same factory. Same quality control. The only difference? The label.
What Exactly Is an Authorized Generic?
An authorized generic is not a copy. It’s not a lookalike. It’s the brand-name drug itself - made by the same company, in the same facility, using the same formula - just sold without the brand name on the bottle. The U.S. Food and Drug Administration (FDA) defines it clearly: it’s the identical product, with the brand name removed from the packaging.
How does this happen? When a drug’s patent expires, the original manufacturer can choose to launch its own generic version. They don’t need to reapply for approval because they’re already selling the drug under a New Drug Application (NDA). This is different from traditional generics, which are made by other companies and must prove they work the same way through bioequivalence studies. Authorized generics skip that step entirely because they’re the original product.
That’s why you won’t find authorized generics listed in the FDA’s Orange Book - the official directory of approved generic drugs. They’re not classified as generics. They’re the brand, in disguise.
Same Ingredients. Same Process. Same Results.
Let’s break down what’s inside the pill. Authorized generics contain the exact same active ingredient - the part that treats your condition - as the brand-name version. But it doesn’t stop there. The inactive ingredients - the fillers, binders, and coatings - are also identical. That matters more than you might think.
Traditional generics sometimes use different inactive ingredients. For most people, that’s fine. But for those with rare allergies or sensitivities, even a tiny change in filler can cause issues. An authorized generic eliminates that risk entirely. No new excipients. No surprises. Just the same formula you’ve been taking for years.
Manufacturing? Same plant. Same machines. Same quality checks. The FDA inspects these facilities the same way - whether they’re making the brand-name version or the authorized generic. The standards don’t change. The inspections don’t change. The batch records don’t change.
And here’s the kicker: authorized generics have to meet the same dissolution standards as the brand. That means the pill breaks down in your body at the same rate, releases the medicine at the same speed, and gets absorbed the same way. There’s no wiggle room. No loopholes. Just exact replication.
Real-World Evidence: Do They Work the Same?
Numbers don’t lie. A major study published in PMC in 2018 tracked over 5,000 patients who switched from brand-name drugs to generics. When they switched to authorized generics, outcomes were nearly identical to staying on the brand. Hospital visits, ER trips, and medication discontinuation rates? No meaningful difference.
Even the slight uptick in ER visits seen with authorized generics - just 8% higher - was likely due to confusion at the pharmacy, not the drug itself. Patients thought they were getting something different. Their anxiety affected how they felt. Not the medicine.
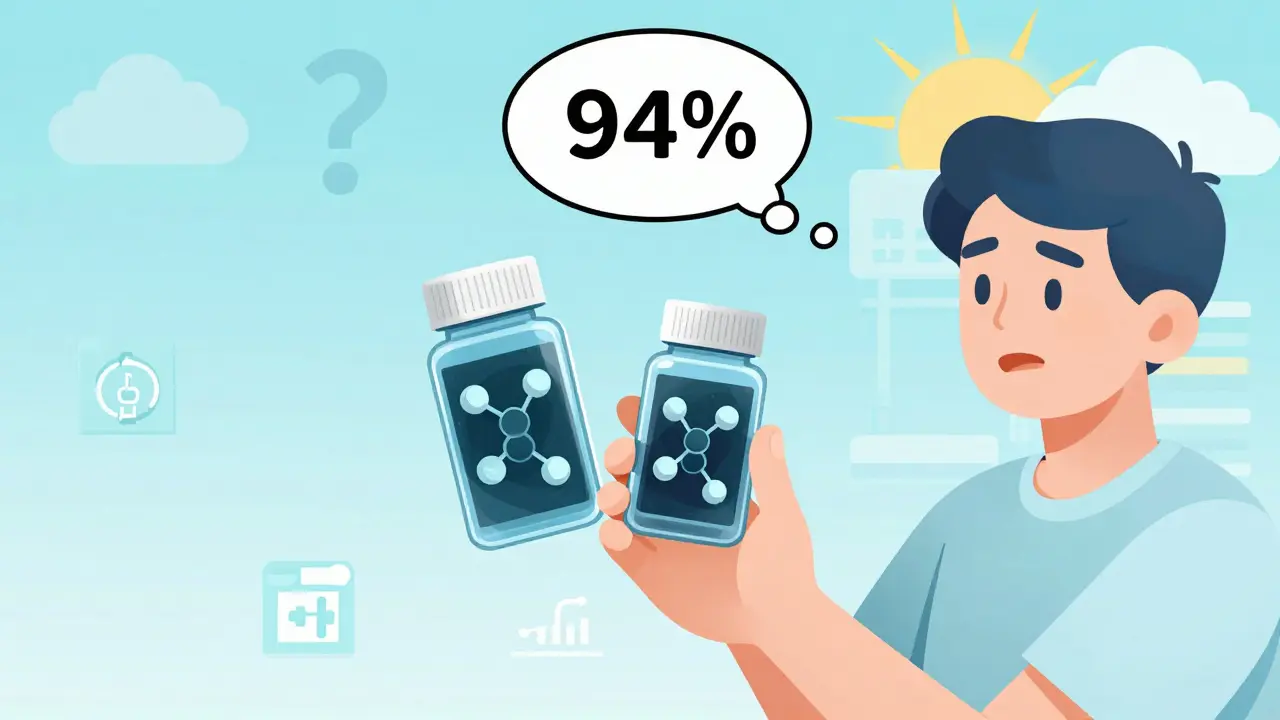
Another study from Kaiser Permanente surveyed over 8,000 patients using authorized generics. Their adherence rates? 94%. That’s higher than the 92% seen with brand-name drugs. People stuck with their meds because they worked - and cost less.
On patient forums like the Asthma and Allergy Foundation of America, 87% of users reported no change in effectiveness when switching from Singulair to its authorized generic. Only 8% noticed minor differences - and those were tied to pill size or dispenser design, not the drug’s performance.

Why Do Authorized Generics Cost More Than Regular Generics?
If they’re the same as the brand, why aren’t they priced the same as traditional generics? Simple: they’re not competing with them. They’re competing with the brand.
Authorized generics typically cost 15-30% less than the brand-name version. But they’re often 10-20% more expensive than traditional generics. Why? Because the original manufacturer controls the supply. They’re using the authorized generic to keep market share after patent expiry. It’s a smart business move - they undercut new generic competitors while still making a profit.
For you? It’s a win. You get the same drug at a lower price than the brand, even if it’s not the cheapest option on the shelf.
What About Insurance and Pharmacy Practices?
Most insurance plans treat authorized generics like traditional generics. That means lower copays. Sometimes, your plan might even prefer them because they’re cheaper than the brand but still reliable.
Pharmacists know the difference. A 2022 survey found that 78% of independent pharmacists consider authorized generics fully interchangeable with the brand - no doctor’s note needed. But here’s the problem: not every pharmacist explains this to patients. Some still think authorized generics are “just another generic.” That’s why you might hear, “This isn’t the real thing,” when it actually is.
If your pharmacist says the authorized generic is different, ask to see the label. Check the manufacturer name. If it matches the brand, you’re holding the same product. Don’t let confusion cost you peace of mind.
Are There Any Downsides?
The biggest issue isn’t the drug. It’s the system.
Because authorized generics look like traditional generics, patients sometimes get switched between versions without knowing. One month you get the authorized generic. Next month, a different generic. The active ingredient is the same, but the inactive ingredients might differ. That can cause confusion - especially for people on multiple medications or with chronic conditions.
Also, not every brand-name drug has an authorized generic. Only about 20-25% of branded drugs offer one after patent expiration. And availability changes fast. A drug might have an authorized generic one year, then lose it the next if the manufacturer decides to pull it.
Still, there’s no evidence these drugs are less safe or less effective. The FDA, the American College of Clinical Pharmacy, and Harvard Medical School all agree: authorized generics are therapeutically equivalent to the brand.
What’s Changing in 2025?
The Inflation Reduction Act of 2022 made changes to Medicare Part D that are pushing more seniors toward lower-cost options. Authorized generics fit perfectly - same efficacy, lower price. By 2027, experts predict they’ll make up 15-18% of the global generic drug market.
The FDA also updated its guidance in 2022 to require stricter lot tracking for authorized generics. That means if there’s ever a recall, they can trace the exact batch back to the factory - just like the brand.
Bottom line: authorized generics aren’t a compromise. They’re the original drug, stripped of marketing. And they’re getting more common, more trusted, and more essential.
What Should You Do?
If you’re on a brand-name drug and your pharmacy offers an authorized generic:
- Ask if it’s available.
- Check the manufacturer name on the bottle - if it matches the brand, you’re good.
- Confirm with your pharmacist: “Is this the exact same pill as the brand?”
- Don’t assume it’s inferior just because it’s cheaper.
- Keep your current prescription as a backup until you’re sure it works for you.
There’s no reason to pay more for the same medicine. Authorized generics aren’t a second choice. They’re the first choice - if you know what you’re getting.


Comments
Greg Quinn
It’s wild how we’ve been conditioned to equate price with quality. I used to think the brand-name pill had some secret sauce-until I switched to the authorized generic for my blood pressure med. Same bottle shape, same imprint, same side effects. Zero difference. Just saved $40 a month. The real drug is the drug, not the logo.
December 28, 2025 at 19:15
Manan Pandya
As someone who manages chronic medication for a family member, I’ve seen the confusion firsthand. Authorized generics aren’t just equivalent-they’re superior in consistency because they’re produced under the same GMP protocols as the brand. The FDA’s oversight doesn’t change just because the label does. This is science, not marketing.
December 29, 2025 at 23:03
Emma Duquemin
Y’all need to stop treating medicine like it’s a fashion brand. I switched my son’s ADHD med to the authorized generic and he didn’t even notice-until I told him we saved $120. He’s 14, not a lab rat. The pill doesn’t care if it’s in a blue box or a white one. The active ingredient? Same. The chemistry? Same. The panic? All in our heads.
December 31, 2025 at 18:22
Aliza Efraimov
I used to be terrified of generics. After my mom had a bad reaction to a regular generic for her thyroid med-different filler, different absorption-I swore off anything that wasn’t brand. Then I found out the authorized generic was the exact same pill she’d been taking for 12 years. No change. No trauma. Just the same medicine without the markup. I cried in the pharmacy aisle.
January 1, 2026 at 19:02
Alex Ronald
For anyone worried about bioequivalence-authorized generics skip the whole testing phase because they’re not generics. They’re the original. The manufacturer doesn’t need to prove equivalence because they’re the ones who made the brand in the first place. It’s like asking if the same cake tastes different when you remove the fancy wrapper.
January 2, 2026 at 20:38
Paige Shipe
Let me be clear: this is a corporate scam. The brand companies are just creating their own ‘generic’ to crush real competitors. They’re not doing this for you-they’re doing it to keep profits. Don’t fall for the ‘same pill’ myth. They’re manipulating the system, and you’re the sucker who thinks you’re saving money.
January 4, 2026 at 04:24
Nisha Marwaha
From a pharmacoeconomic standpoint, authorized generics represent a unique regulatory artifact-essentially an NDA product repackaged under ANDA-like distribution channels. The absence of bioequivalence studies is not a deficiency; it’s a validation of identity. The excipient matrix remains invariant, and dissolution profiles are preserved under the original NDA’s specifications.
January 5, 2026 at 04:38
David Chase
AMERICA NEEDS TO STOP BEING SO GULLIBLE!! 🇺🇸 The brand-name drug is MADE IN THE USA!! The ‘generic’? Probably made in some factory in China with 12-year-olds stirring the powder!! 😡 This ‘authorized’ crap is just a Trojan horse for foreign pharma!! DON’T BE FOOLED!!
January 5, 2026 at 22:32
Teresa Rodriguez leon
My doctor told me to switch and I cried for three days. I felt like I was being forced to take something cheap. But then I took it. And I didn’t die. And my blood pressure didn’t spike. And I still have my dignity. I just wish someone had told me this sooner instead of letting me suffer in fear.
January 6, 2026 at 09:20
Tamar Dunlop
It is, indeed, a matter of profound significance that the regulatory architecture underpinning pharmaceutical efficacy permits such a transparent alignment between branded and authorized generic formulations. The integrity of the manufacturing process, preserved under identical Good Manufacturing Practices, renders the distinction purely semiotic-a matter of nomenclature, not pharmacology.
January 6, 2026 at 17:25
Kevin Lopez
Authorized generic = brand. Not generic. Don’t confuse the two. End of story.
January 7, 2026 at 09:37