When you pick up a generic pill, you expect it to do the same thing as the brand-name version—and bioequivalence standards, the scientific rules that prove generic drugs perform the same way in the body as their brand-name counterparts. Also known as therapeutic equivalence, these standards are the invisible gatekeepers that decide whether a cheaper pill can legally replace an expensive one. Without them, generic drugs could be useless—or worse, dangerous. The FDA doesn’t just check the ingredients; it demands proof that the drug gets into your bloodstream at the same rate and amount, so your blood pressure, blood sugar, or seizure control doesn’t flip upside down when you switch.
These standards aren’t just paperwork. They’re built on real human studies, often with healthy volunteers who swallow identical doses of brand and generic versions while researchers track how the drug moves through their bodies. If the numbers match within tight limits—usually 80% to 125% of the original drug’s absorption—it passes. But here’s the catch: FDA approval, the official process that grants permission for a generic drug to be sold in the U.S. doesn’t end when bioequivalence is proven. Many drugs sit in limbo because of patent fights, manufacturing delays, or economic incentives that favor the brand. That’s why you might see a generic version approved for years but still not on your pharmacy shelf.
And it’s not just about the active ingredient. generic drugs, medications that contain the same active ingredient as a brand-name drug but are sold under a different name after the patent expires can still differ in fillers, coatings, or release mechanisms. Those might not affect bioequivalence, but they can change how the pill feels in your stomach or how fast it dissolves. For most people, it doesn’t matter. For someone on narrow-therapeutic-index drugs like warfarin or lithium, even small differences can be risky. That’s why some doctors still prescribe brand names—even when generics exist.
Behind every generic drug you take is a chain of science, regulation, and business decisions. Bioequivalence standards are the backbone of affordable medicine, but they’re not foolproof. Some drugs slip through gaps in enforcement. Others get stuck in legal battles that delay access for years. And patients rarely know why their insurance won’t cover the generic they were promised. The posts below dig into these hidden layers—how the FDA monitors generics after approval, why tentative approvals don’t mean market access, and how drug shortages and formulary rules shape what ends up in your medicine cabinet. You’ll see real examples of how these standards play out in everyday care, from cancer treatments to blood pressure pills. What you learn might change how you think about the pills you swallow every day.
Posted by
Paul Fletcher
8 Comments
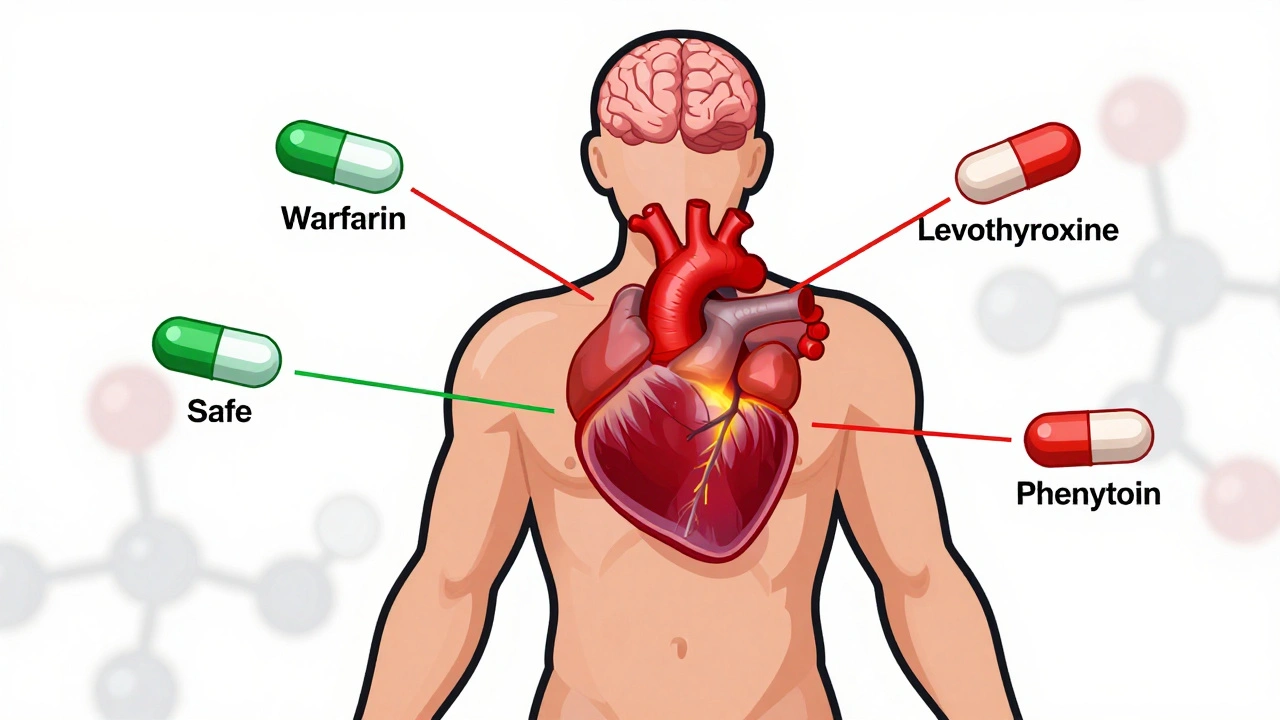
NTI generics require stricter regulation than standard generics due to narrow margins between effective and toxic doses. This article explores how the FDA, EMA, Canada, and Japan differ in their approaches, what’s changing in 2025, and why prescribers must stay cautious.
read more