When you switch from a brand-name drug to a generic, you expect the same results. But for drugs with a Narrow Therapeutic Index, a category of medications where small changes in dose can cause serious harm or treatment failure. Also known as NTI drugs, these include blood thinners like warfarin, seizure meds like phenytoin, and thyroid hormones like levothyroxine. The FDA doesn’t treat them like regular generics. Their FDA NTI guidelines demand tighter controls—because a 10% difference in absorption could mean a seizure, a stroke, or worse.
These guidelines exist because NTI drugs leave almost no room for error. A generic version must match the brand not just in active ingredient, but in how fast and completely it enters your bloodstream. The FDA requires bioequivalence studies with stricter limits than for other drugs. This isn’t just paperwork—it’s a safety net. If a generic fails to meet these standards, it won’t get approved. And if it does, the FDA still watches it closely through post-market monitoring, just like they do with all drugs. You might not know it, but your pharmacist’s decision to swap your levothyroxine brand for a generic isn’t random. It’s guided by these rules. And if you’ve ever been told to stick with one brand, it’s likely because your doctor knows the NTI rules apply to your medicine.
Related entities like bioequivalence, the scientific process of proving a generic drug performs the same way in the body as the brand, and therapeutic equivalence, the FDA’s official rating system that tells pharmacists which generics can be swapped safely are built on these NTI standards. Even ANDA applications, the filings generic manufacturers submit to the FDA for approval, get extra scrutiny for NTI drugs. That’s why you see fewer generic options for these meds—they’re harder and more expensive to get approved. But when they do make it to market, you can trust they’ve passed a higher bar.
What you’ll find in the posts below aren’t just random articles about drugs—they’re a practical guide to how these rules shape what’s in your medicine cabinet. From how the FDA tracks safety after approval to why some generics never reach shelves, each piece connects back to the same truth: when a drug has a narrow therapeutic index, the system doesn’t cut corners. You’ll learn why switching meds isn’t always safe, how to talk to your doctor about alternatives, and what to watch for if your prescription changes. This isn’t theory. It’s about the pills you take every day—and why the rules around them matter more than you think.
Posted by
Paul Fletcher
8 Comments
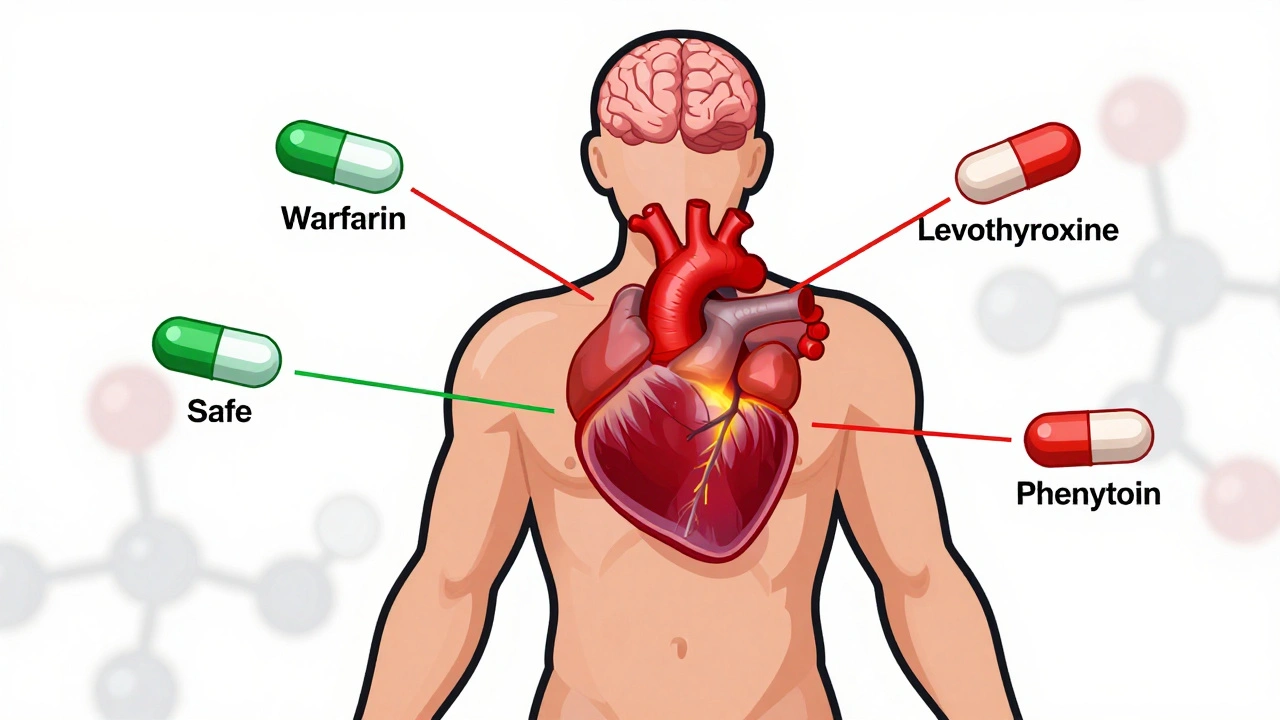
NTI generics require stricter regulation than standard generics due to narrow margins between effective and toxic doses. This article explores how the FDA, EMA, Canada, and Japan differ in their approaches, what’s changing in 2025, and why prescribers must stay cautious.
read more