When you pick up a generic pill at the pharmacy, you’re seeing the result of federal drug policy, the set of laws and regulations that govern how drugs are tested, approved, priced, and monitored in the United States. Also known as pharmaceutical regulation, it’s not just about safety—it’s about who gets access, how much it costs, and whether a life-saving drug reaches the right person at the right time. This isn’t abstract bureaucracy. It’s the reason some generics sit on shelves for years after being scientifically approved, why your insurance only covers certain brands, and why a drug you rely on might suddenly disappear from your pharmacy.
The FDA, the U.S. agency responsible for evaluating drug safety and efficacy before they hit the market doesn’t stop working once a drug is approved. Its post-marketing pharmacovigilance, the ongoing monitoring of drugs after they’re sold to the public catches side effects that clinical trials miss—like rare heart issues with mRNA vaccines or dangerous drops in blood sugar from sulfonylureas. But the FDA doesn’t control everything. Prescription regulations, the rules set by Congress and federal agencies that dictate how drugs are prescribed, distributed, and paid for are shaped by insurers, pharmacy benefit managers, and patent laws. That’s why a generic drug can be scientifically ready but still blocked by legal battles over patents, or why your copay for a generic is still too high even though it costs pennies to make.
These systems don’t work in isolation. A delay in generic drugs, affordable versions of brand-name medications that must meet the same quality standards approval ties directly to patent litigation, which is a legal tactic, not a scientific one. Meanwhile, the drug safety, the continuous process of identifying and responding to harmful side effects after a drug is in wide use system relies on patient reports through the FAERS database—meaning your call to your doctor about a strange reaction helps shape national policy. And when insurers push preferred generic lists to cut costs, they’re acting under federal guidelines that reward lower prices—but sometimes at the cost of patient choice.
What you’ll find below isn’t just a list of articles. It’s a map of how federal drug policy touches your life—from the tiny print on your prescription label to the hidden delays blocking cheaper medicines. You’ll see how the Purple Book tracks biosimilars, how smoking changes clozapine levels, why some generics never reach shelves, and how real-world data catches dangers clinical trials miss. These aren’t theoretical debates. They’re real barriers, real savings, and real risks that shape your health every day. Read on to understand not just what’s in your medicine cabinet, but why it got there—and what you can do about it.
Posted by
Paul Fletcher
8 Comments
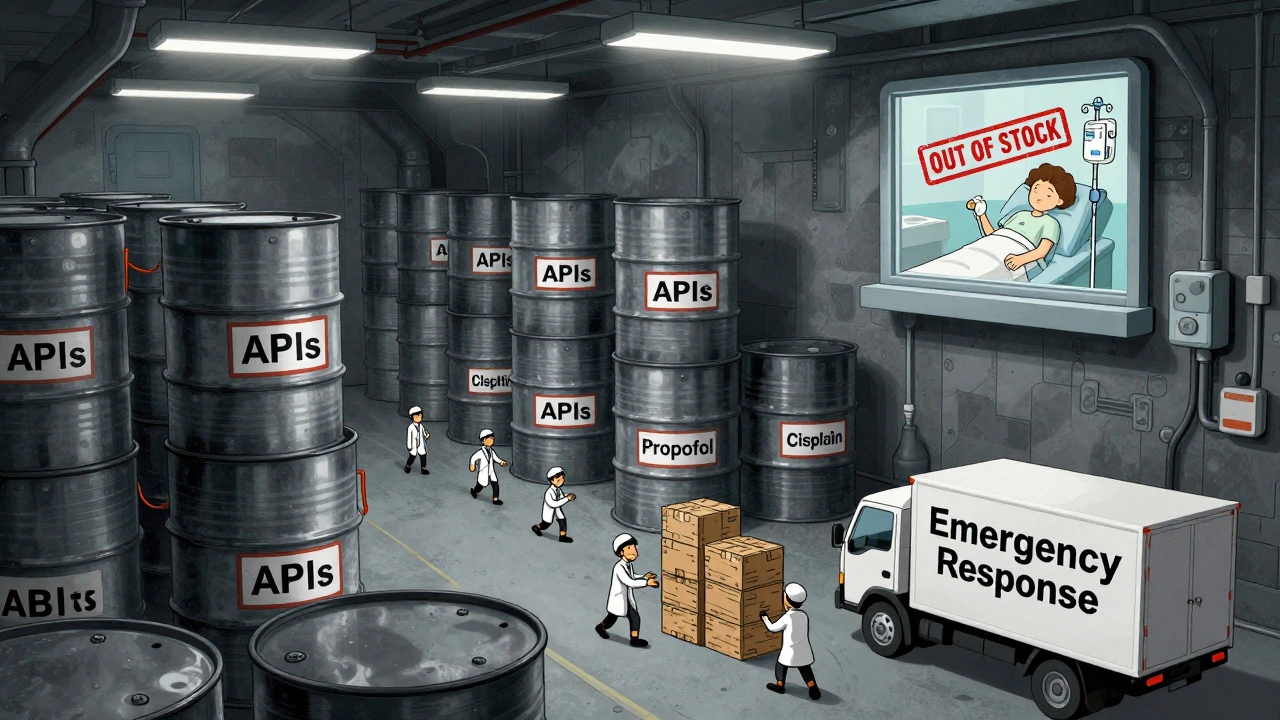
In 2025, the U.S. government expanded its drug shortage response with a new API stockpile program, but gaps in enforcement, funding, and economic incentives leave hospitals and patients vulnerable to ongoing shortages of critical medications.
read more