When you pick up a generic drug, a lower-cost version of a brand-name medication that contains the same active ingredient, strength, and dosage form. Also known as generic medication, it is legally required to work just like the original—no exceptions. But how do we know it’s truly the same? That’s where generic drug regulation, the system of rules and oversight enforced by the FDA to ensure generic drugs meet strict standards for safety, quality, and effectiveness comes in. It’s not just paperwork. It’s a chain of checks that starts in the lab and ends with your medicine cabinet.
Before a generic drug can be sold, the manufacturer must prove it delivers the same amount of active ingredient into your bloodstream at the same rate as the brand-name version. This is called bioequivalence. The FDA doesn’t just accept claims—they test it themselves. But approval isn’t the end. Many generics get tentative approval, a status given when a drug is scientifically ready but blocked by patents or exclusivity rights, meaning it sits on the shelf even though it’s ready to go. And once it’s on the market? The FDA keeps watching. Through systems like FAERS, the FDA’s database for collecting and analyzing reports of adverse events from patients and doctors, they track side effects, contamination, and unexpected reactions. Real people report problems. AI helps spot patterns. Inspectors show up at factories without warning. This isn’t theoretical—it’s how they caught unsafe batches and fixed them before more people got hurt.
Regulation isn’t perfect. Delays happen because of legal battles over patents, not science. Some generics never reach shelves because the profit isn’t high enough. But the system works more often than not. The FDA doesn’t treat generics like second-class drugs. They’re held to the same standards, monitored the same way, and trusted by millions daily. What changes is how you access them—through insurance formularies, pharmacy delivery, or copay assistance programs that make these regulated drugs affordable. You don’t need to guess if your generic is safe. The system was built so you wouldn’t have to.
Below, you’ll find real stories and facts about how generic drugs move from approval to your pillbox—and what happens when things go wrong. From how smoking affects your generic antipsychotic to why some drugs sit in limbo for years, these posts show the human side of regulation. You’re not just reading about policy. You’re learning how to get the right medicine, safely and affordably.
Posted by
Paul Fletcher
8 Comments
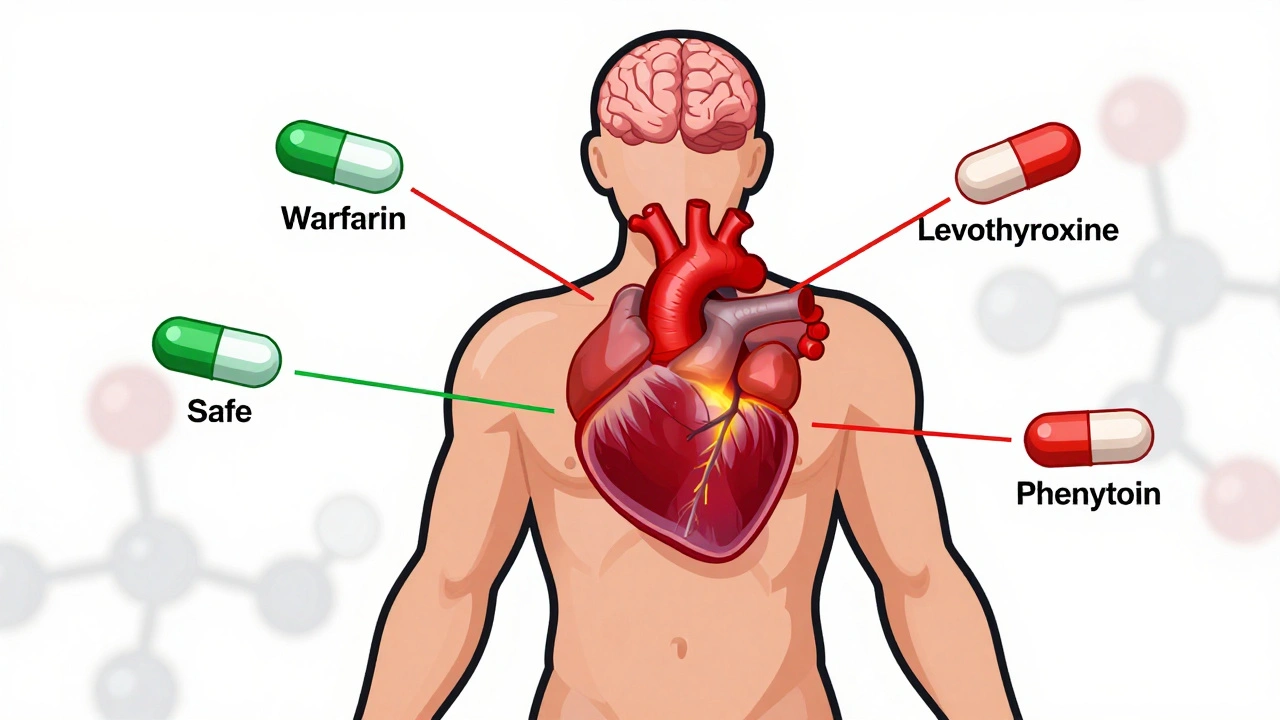
NTI generics require stricter regulation than standard generics due to narrow margins between effective and toxic doses. This article explores how the FDA, EMA, Canada, and Japan differ in their approaches, what’s changing in 2025, and why prescribers must stay cautious.
read more