When you hear brand name drugs, the original versions of medications developed and marketed by pharmaceutical companies under a proprietary name. Also known as innovator drugs, they’re the first to hit the market after years of research and clinical trials. These are the pills you see advertised on TV — the ones with catchy names like Lipitor, Prozac, or Humira. They’re not just labels. They represent patents, investments, and sometimes, a huge price gap compared to their generic counterparts.
Behind every brand name drug, a medication approved by the FDA after rigorous testing for safety and effectiveness is a decade or more of development. Companies spend billions to prove these drugs work, which is why they charge more — they’re recovering costs and making a profit before generics can enter the market. Once the patent expires, other manufacturers can copy the active ingredient and sell it as a generic drug, a bioequivalent version of the brand name drug with the same dosage, strength, and intended use. Also known as generic medication, these versions often cost 80% to 95% less. But here’s the catch: just because they’re cheaper doesn’t mean they’re different in how they work. The FDA requires generics to meet the same standards for quality, safety, and effectiveness as the original.
Still, not all brand name drugs have direct generic equivalents. Some are complex biologics, like those used in cancer or autoimmune treatments, where even small changes in manufacturing can affect how the drug behaves in your body. That’s where biosimilars, highly similar versions of biologic drugs approved under special FDA pathways come in. They’re not exact copies, but they’re close enough to be considered safe and effective alternatives. The FDA tracks these in the Purple Book, and insurers are slowly starting to favor them over the original brand names — but not always.
Why does this matter to you? Because your prescription might be listed as a brand name drug, but your insurance could push you toward a generic — or even a biosimilar — to save money. Sometimes, that switch works perfectly. Other times, you might notice side effects or feel the medication doesn’t work the same. That’s not always about quality. It could be about inactive ingredients, how the drug is absorbed, or even psychological factors. The key is to talk to your doctor before switching. Don’t assume generics are always better or worse — just different.
And while brand name drugs often get the spotlight, most of the real-world drug safety data comes from how these medications perform after they’re widely used. That’s where post-marketing pharmacovigilance, the ongoing monitoring of drug safety after approval steps in. The FDA watches for unexpected side effects, drug interactions, and misuse — whether the drug is brand or generic. Your report of a problem matters more than you think.
What you’ll find below are real, practical stories about how brand name drugs interact with your life — from why insurers prefer generics, to how smoking can change your medication levels, to why some generics never make it to pharmacy shelves. These aren’t abstract debates. They’re decisions that affect your wallet, your health, and your daily routine. Whether you’re paying out of pocket, dealing with insurance denials, or just wondering why your doctor prescribed one version over another, the answers are here.
Posted by
Jenny Garner
11 Comments

Authorized generics are the exact same drug as brand-name medications - same ingredients, same factory, same results. Learn why they’re just as effective, often cheaper, and why you should consider them.
read morePosted by
Jenny Garner
8 Comments
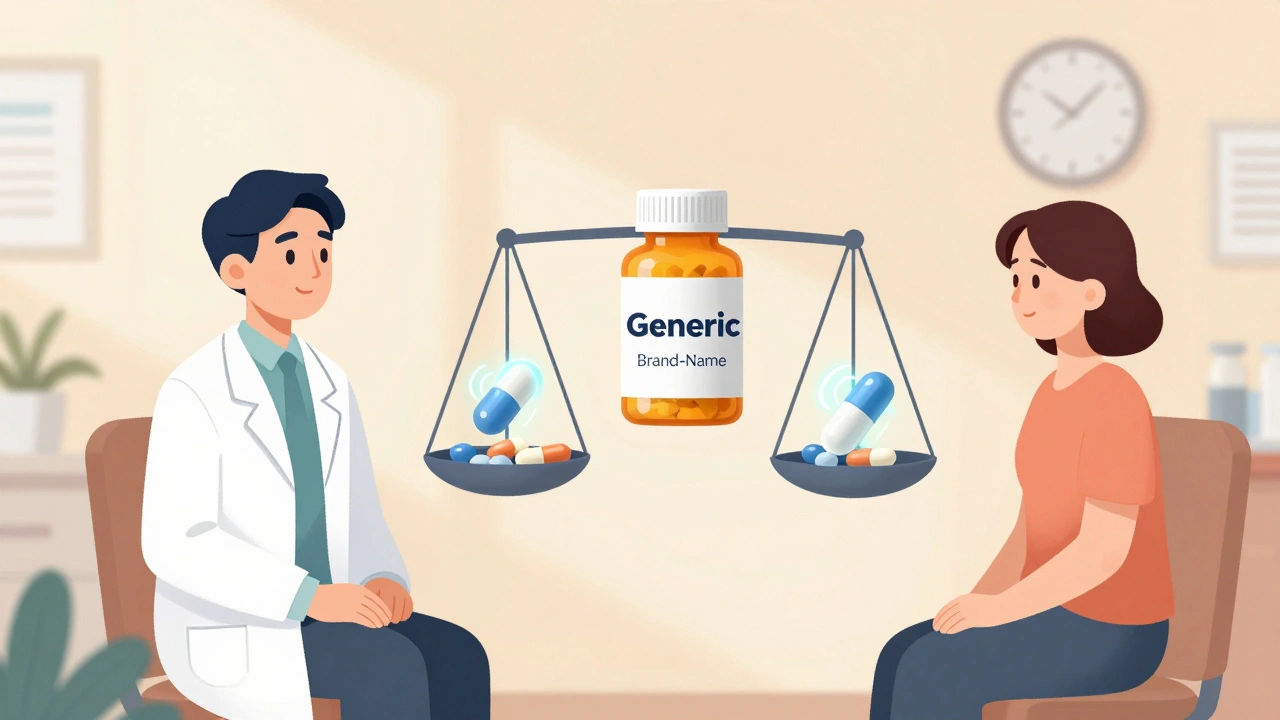
Learn how to talk to your doctor about generic vs. brand-name medications-when generics work just as well, when to ask for the brand, and how to save money without risking your health.
read more