Posted by
Paul Fletcher
8 Comments
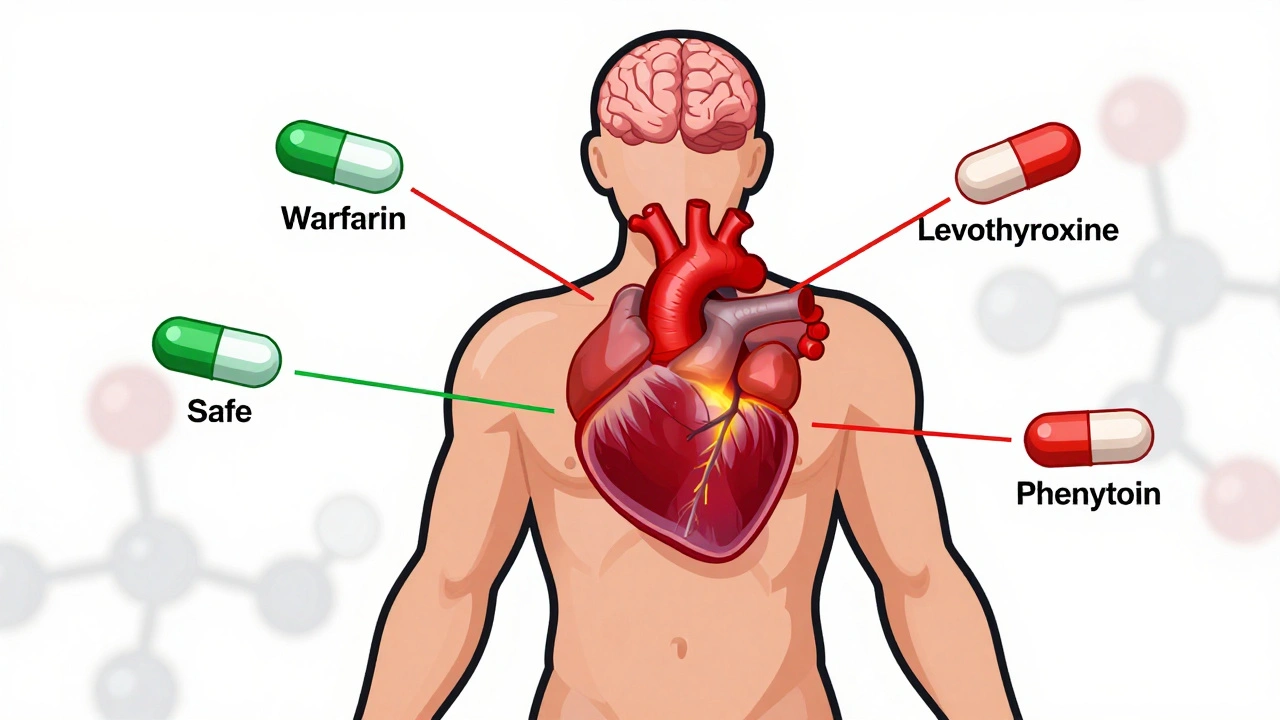
NTI generics require stricter regulation than standard generics due to narrow margins between effective and toxic doses. This article explores how the FDA, EMA, Canada, and Japan differ in their approaches, what’s changing in 2025, and why prescribers must stay cautious.
read morePosted by
Paul Fletcher
8 Comments
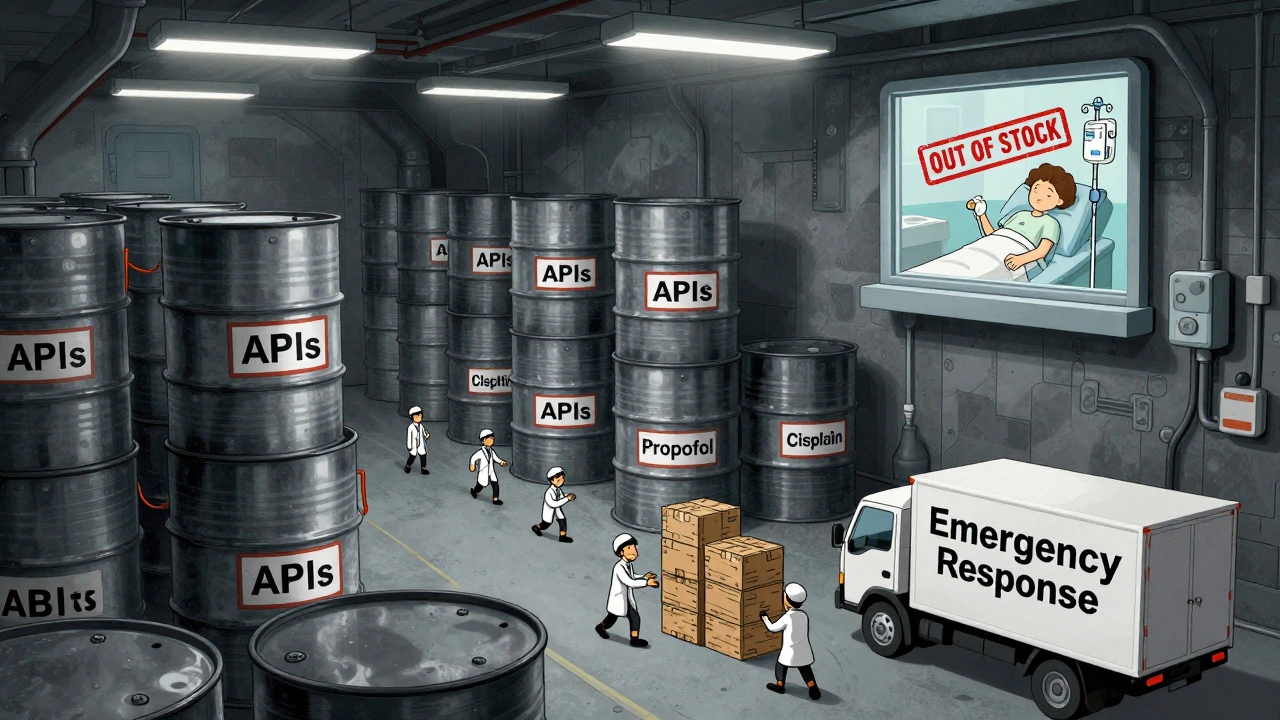
In 2025, the U.S. government expanded its drug shortage response with a new API stockpile program, but gaps in enforcement, funding, and economic incentives leave hospitals and patients vulnerable to ongoing shortages of critical medications.
read morePosted by
Paul Fletcher
12 Comments

Tentative FDA approval for generics means the drug is scientifically ready - but legal, manufacturing, or economic barriers often block its market launch. Here's why so many generics never reach patients.
read morePosted by
Jenny Garner
13 Comments
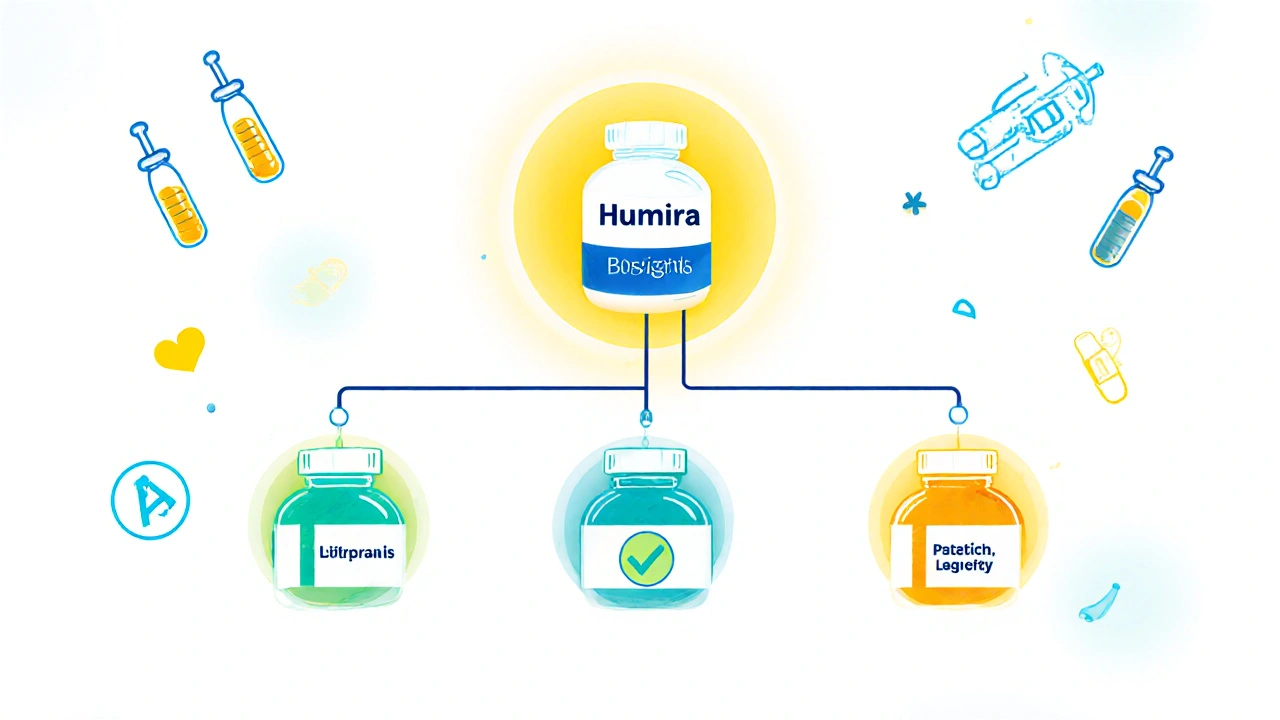
The FDA's Purple Book is the official source for tracking approved biological products, biosimilars, and interchangeable biologics. Learn how it works, what interchangeability really means, and how it affects your prescriptions.
read morePosted by
Paul Fletcher
16 Comments

Discover reliable alternatives to WebMD for your health information needs. From the well-respected Mayo Clinic to the government-backed NIH, these platforms offer credible and comprehensive resources. Each alternative provides unique features and expert-reviewed content to help users make informed health decisions.
read more