Posted by
Paul Fletcher
8 Comments
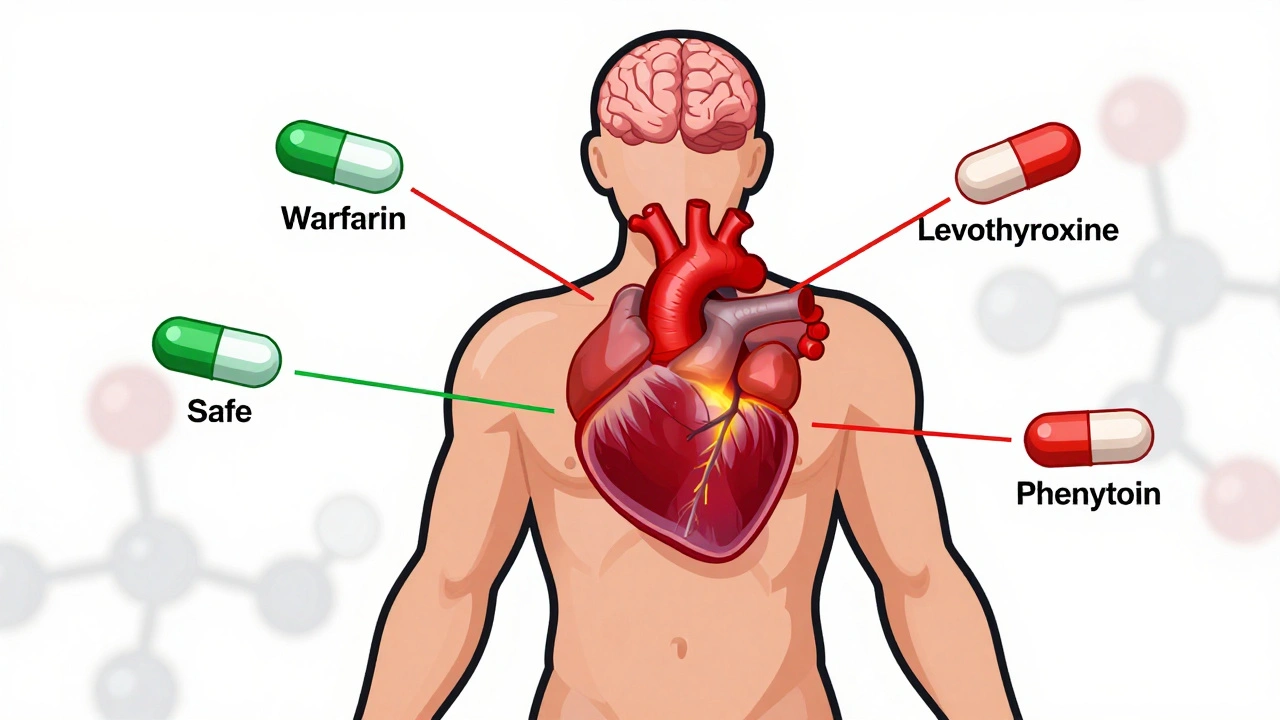
NTI generics require stricter regulation than standard generics due to narrow margins between effective and toxic doses. This article explores how the FDA, EMA, Canada, and Japan differ in their approaches, what’s changing in 2025, and why prescribers must stay cautious.
read morePosted by
Paul Fletcher
11 Comments
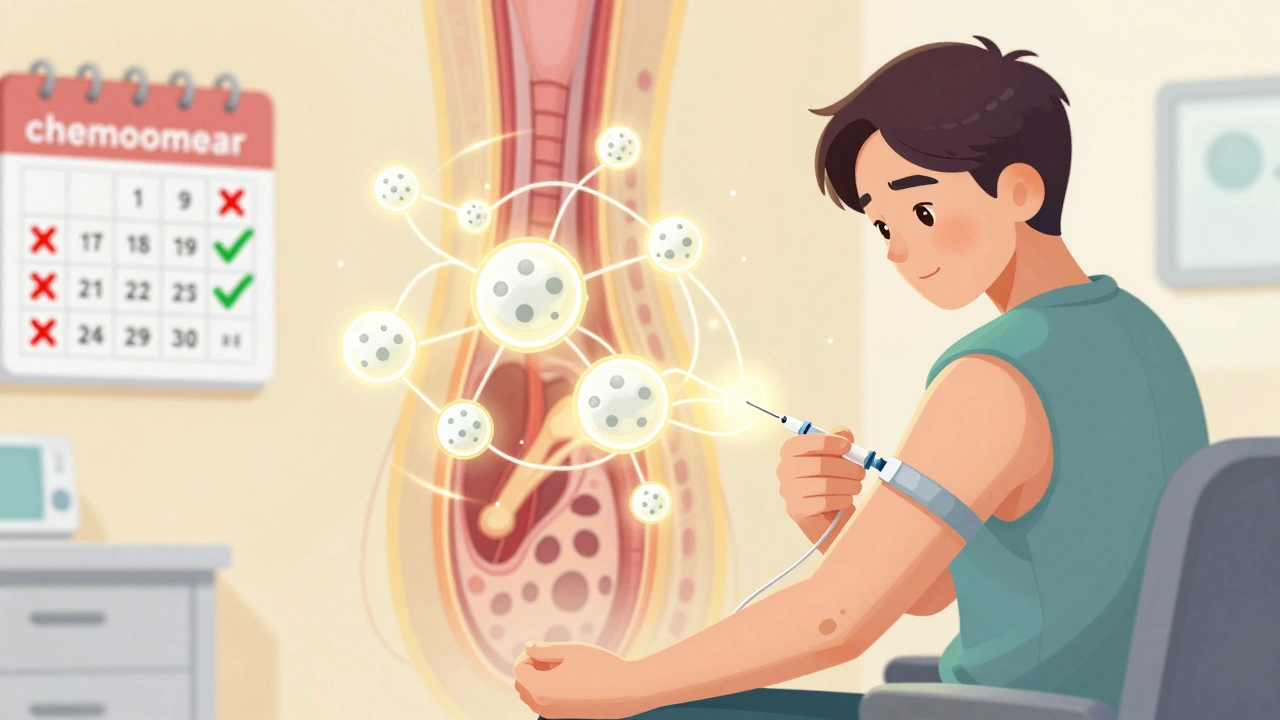
Supportive care in cancer uses growth factors, antiemetics, and pain relief to manage treatment side effects, improve survival, and keep patients on track. Learn how these evidence-based tools work and why access remains unequal.
read morePosted by
Paul Fletcher
8 Comments
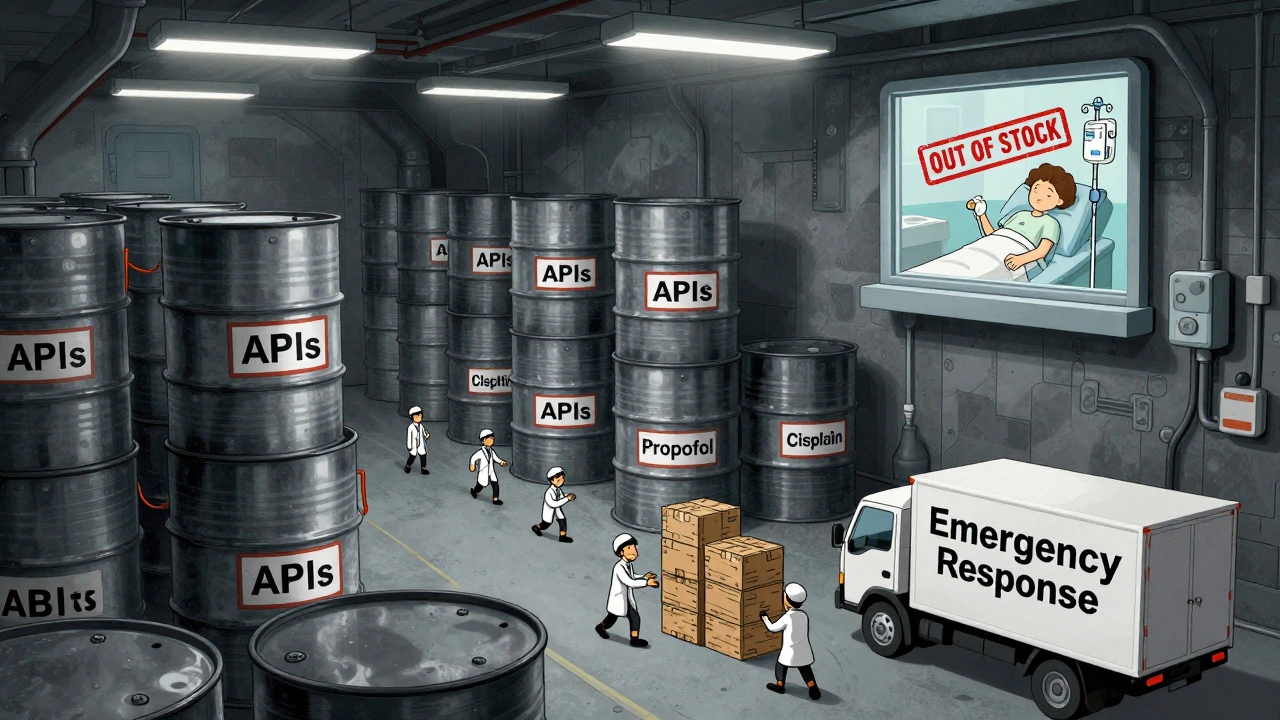
In 2025, the U.S. government expanded its drug shortage response with a new API stockpile program, but gaps in enforcement, funding, and economic incentives leave hospitals and patients vulnerable to ongoing shortages of critical medications.
read morePosted by
Paul Fletcher
12 Comments

Tentative FDA approval for generics means the drug is scientifically ready - but legal, manufacturing, or economic barriers often block its market launch. Here's why so many generics never reach patients.
read morePosted by
Paul Fletcher
9 Comments

The FDA doesn't stop monitoring generic drugs after approval. Using real-time data, inspections, and patient reports, it tracks safety issues long after these affordable medications hit the market. Here's how it works.
read morePosted by
Paul Fletcher
16 Comments
Checkpoint inhibitors and CAR-T cell therapy are transforming cancer treatment by harnessing the immune system. Learn how they work, who benefits most, their side effects, and why access remains unequal.
read more