Posted by
Jenny Garner
10 Comments
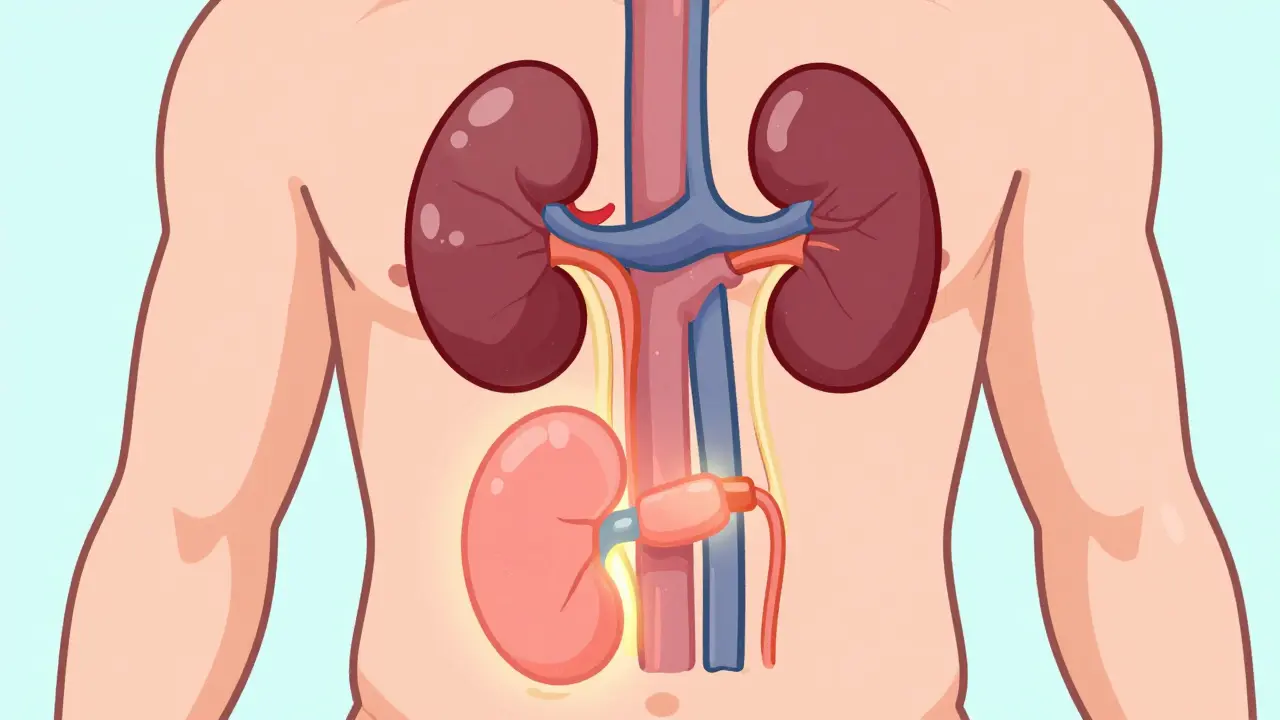
A kidney transplant offers a better quality of life and longer survival than dialysis for people with end-stage kidney disease. Learn who qualifies, what the surgery involves, and how to manage life with a new kidney.
read morePosted by
Jenny Garner
11 Comments

Authorized generics are the exact same drug as brand-name medications - same ingredients, same factory, same results. Learn why they’re just as effective, often cheaper, and why you should consider them.
read morePosted by
Paul Fletcher
12 Comments
PCSK9 inhibitors and statins both lower LDL cholesterol, but they work differently and have very different side effects. Learn who benefits most from each, how they compare in safety and effectiveness, and what to do if statins aren’t working for you.
read morePosted by
Jenny Garner
14 Comments

Only specific FDA-approved medications should be flushed down the toilet - mostly powerful opioids and sedatives that can kill with one dose. Learn which ones, why, and when flushing is the safest option.
read morePosted by
Paul Fletcher
9 Comments
Real-world evidence from patient registries and claims data is now essential for monitoring drug safety after approval. Learn how these sources help regulators catch rare side effects and ensure medications are safe for millions.
read morePosted by
Jenny Garner
13 Comments

Medication adherence means actively choosing to follow a treatment plan with your provider's support - not just obeying orders. Learn why this shift from compliance to adherence saves lives, reduces hospital visits, and improves outcomes for chronic conditions.
read morePosted by
Jenny Garner
14 Comments

Hand hygiene is the most effective way to prevent infections at home. Learn the science-backed steps for washing hands properly, when to use sanitizer, and how to protect your family from germs-without spending a lot.
read morePosted by
Jenny Garner
9 Comments

Pharmacist counseling scripts are structured tools to ensure patients understand how to take their medications safely. Learn how these training materials improve adherence, meet legal requirements, and adapt to real-world pharmacy settings.
read morePosted by
Paul Fletcher
15 Comments
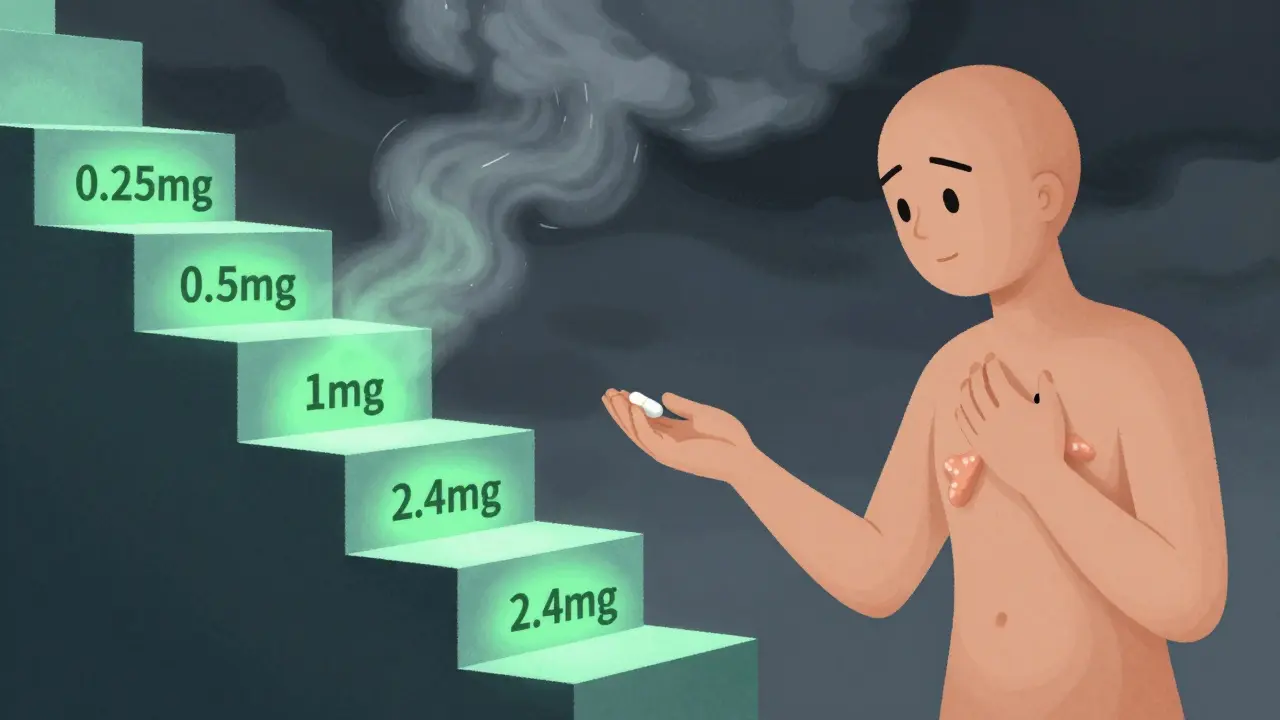
Slow up-titration schedules help your body adjust to new medications, reducing side effects and improving long-term adherence. Learn how it works for GLP-1 agonists, beta-blockers, and more.
read morePosted by
Paul Fletcher
15 Comments

Track your body's response when switching to generic medications with a simple medication journal. Learn what to record, when it matters most, and how to use your notes to get better care.
read more